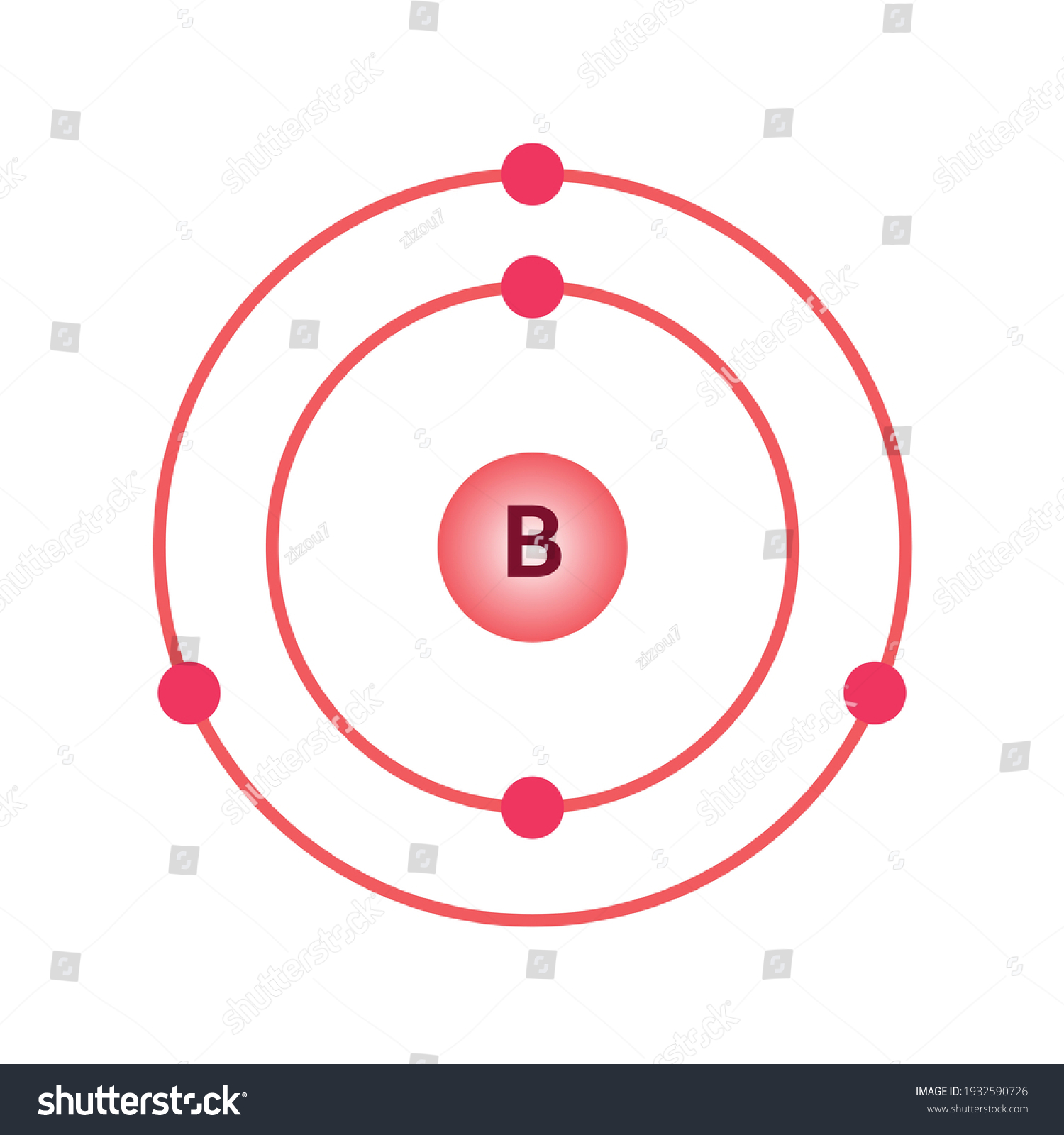
Does Boron Share Electrons . Valence electrons are the electrons in the highest occupied principal energy level of an atom. In order to achieve a stable electron configuration, boron can either lose three electrons or gain five electrons. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic. Borax ore (known as tincal); And with calcium in colemanite (cab 3 o 4 (oh) 4. Boron has a charge of 5. However, the only facet of an atom that dictates anything about the number of valence electrons is its nuclear charge. The atomic number of each element increases by one, reading from left to right. Blockelements are organised into blocks by the orbital type in. In the second period elements, the two electrons in.
from www.shutterstock.com
With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic. And with calcium in colemanite (cab 3 o 4 (oh) 4. Boron has a charge of 5. The atomic number of each element increases by one, reading from left to right. Blockelements are organised into blocks by the orbital type in. In the second period elements, the two electrons in. However, the only facet of an atom that dictates anything about the number of valence electrons is its nuclear charge. Valence electrons are the electrons in the highest occupied principal energy level of an atom. Borax ore (known as tincal); In order to achieve a stable electron configuration, boron can either lose three electrons or gain five electrons.
Bohr Model Boron Atom Electron Structure Stock Vector (Royalty Free
Does Boron Share Electrons However, the only facet of an atom that dictates anything about the number of valence electrons is its nuclear charge. However, the only facet of an atom that dictates anything about the number of valence electrons is its nuclear charge. Boron has a charge of 5. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic. And with calcium in colemanite (cab 3 o 4 (oh) 4. The atomic number of each element increases by one, reading from left to right. Valence electrons are the electrons in the highest occupied principal energy level of an atom. Blockelements are organised into blocks by the orbital type in. In the second period elements, the two electrons in. In order to achieve a stable electron configuration, boron can either lose three electrons or gain five electrons. Borax ore (known as tincal);
From autoctrls.com
Understanding Boron Electron Dot Diagram A Comprehensive Guide Does Boron Share Electrons In the second period elements, the two electrons in. Blockelements are organised into blocks by the orbital type in. In order to achieve a stable electron configuration, boron can either lose three electrons or gain five electrons. And with calcium in colemanite (cab 3 o 4 (oh) 4. Valence electrons are the electrons in the highest occupied principal energy level. Does Boron Share Electrons.
From periodictable.me
How To Find The Boron Electron Configuration (B) Does Boron Share Electrons The atomic number of each element increases by one, reading from left to right. Valence electrons are the electrons in the highest occupied principal energy level of an atom. However, the only facet of an atom that dictates anything about the number of valence electrons is its nuclear charge. In order to achieve a stable electron configuration, boron can either. Does Boron Share Electrons.
From www.pinterest.co.kr
Electron Configuration For Boron spdf Electronic Configuration Does Boron Share Electrons Blockelements are organised into blocks by the orbital type in. In the second period elements, the two electrons in. Valence electrons are the electrons in the highest occupied principal energy level of an atom. Boron has a charge of 5. The atomic number of each element increases by one, reading from left to right. And with calcium in colemanite (cab. Does Boron Share Electrons.
From www.youtube.com
How many valence electrons does boron have?How to find the valence Does Boron Share Electrons Borax ore (known as tincal); Blockelements are organised into blocks by the orbital type in. The atomic number of each element increases by one, reading from left to right. And with calcium in colemanite (cab 3 o 4 (oh) 4. Valence electrons are the electrons in the highest occupied principal energy level of an atom. With its high ionization energy,. Does Boron Share Electrons.
From borates.today
Boron Electron Valence Borates Today Does Boron Share Electrons Boron has a charge of 5. However, the only facet of an atom that dictates anything about the number of valence electrons is its nuclear charge. Borax ore (known as tincal); With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic. In the second period elements, the two electrons in.. Does Boron Share Electrons.
From www.pinterest.com.au
FicheiroElectron shell 005 Boron.svg Wikipédia, a enciclopédia livre Does Boron Share Electrons Boron has a charge of 5. However, the only facet of an atom that dictates anything about the number of valence electrons is its nuclear charge. Blockelements are organised into blocks by the orbital type in. And with calcium in colemanite (cab 3 o 4 (oh) 4. In the second period elements, the two electrons in. Valence electrons are the. Does Boron Share Electrons.
From www.alamy.com
Periodic Table of the Elements, Shell Structure of Boron B Electrons Does Boron Share Electrons Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in. The atomic number of each element increases by one, reading from left to right. However, the only facet of an atom that dictates anything about the number of valence electrons is its nuclear charge. Blockelements are. Does Boron Share Electrons.
From www.dreamstime.com
Boron Atom Bohr Model With Proton, Neutron And Electron Stock Does Boron Share Electrons In order to achieve a stable electron configuration, boron can either lose three electrons or gain five electrons. Borax ore (known as tincal); Boron has a charge of 5. And with calcium in colemanite (cab 3 o 4 (oh) 4. Blockelements are organised into blocks by the orbital type in. Valence electrons are the electrons in the highest occupied principal. Does Boron Share Electrons.
From techiescientist.com
Boron Bohr Model Diagram, Steps To Draw Techiescientist Does Boron Share Electrons In the second period elements, the two electrons in. Borax ore (known as tincal); Blockelements are organised into blocks by the orbital type in. Boron has a charge of 5. In order to achieve a stable electron configuration, boron can either lose three electrons or gain five electrons. With its high ionization energy, low electron affinity, low electronegativity, and small. Does Boron Share Electrons.
From www.shutterstock.com
Bohr Model Boron Atom Electron Structure Stock Vector (Royalty Free Does Boron Share Electrons In the second period elements, the two electrons in. The atomic number of each element increases by one, reading from left to right. Boron has a charge of 5. However, the only facet of an atom that dictates anything about the number of valence electrons is its nuclear charge. And with calcium in colemanite (cab 3 o 4 (oh) 4.. Does Boron Share Electrons.
From www.shutterstock.com
Boron Atomic Structure Has Atomic Number Stock Vector (Royalty Free Does Boron Share Electrons Borax ore (known as tincal); However, the only facet of an atom that dictates anything about the number of valence electrons is its nuclear charge. In the second period elements, the two electrons in. Blockelements are organised into blocks by the orbital type in. The atomic number of each element increases by one, reading from left to right. And with. Does Boron Share Electrons.
From mungfali.com
Boron Electron Dot Diagram Does Boron Share Electrons Valence electrons are the electrons in the highest occupied principal energy level of an atom. However, the only facet of an atom that dictates anything about the number of valence electrons is its nuclear charge. In the second period elements, the two electrons in. In order to achieve a stable electron configuration, boron can either lose three electrons or gain. Does Boron Share Electrons.
From fr.dreamstime.com
Atom of Boron with Detailed Core and Its 5 Electrons with Atoms Does Boron Share Electrons In the second period elements, the two electrons in. Valence electrons are the electrons in the highest occupied principal energy level of an atom. Blockelements are organised into blocks by the orbital type in. The atomic number of each element increases by one, reading from left to right. And with calcium in colemanite (cab 3 o 4 (oh) 4. However,. Does Boron Share Electrons.
From ar.pinterest.com
Boron Art Print by Carlos Clarivan Atomic structure, Boron, Atom Does Boron Share Electrons In the second period elements, the two electrons in. Boron has a charge of 5. Blockelements are organised into blocks by the orbital type in. Borax ore (known as tincal); With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic. However, the only facet of an atom that dictates anything. Does Boron Share Electrons.
From www.animalia-life.club
Boron Protons Neutrons Electrons Does Boron Share Electrons In order to achieve a stable electron configuration, boron can either lose three electrons or gain five electrons. However, the only facet of an atom that dictates anything about the number of valence electrons is its nuclear charge. Valence electrons are the electrons in the highest occupied principal energy level of an atom. With its high ionization energy, low electron. Does Boron Share Electrons.
From material-properties.org
Boron Periodic Table and Atomic Properties Does Boron Share Electrons The atomic number of each element increases by one, reading from left to right. Borax ore (known as tincal); With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic. In order to achieve a stable electron configuration, boron can either lose three electrons or gain five electrons. And with calcium. Does Boron Share Electrons.
From littleeagles.edu.vn
Boron Valence Electrons Boron Valency (B) With Dot Diagram Does Boron Share Electrons Valence electrons are the electrons in the highest occupied principal energy level of an atom. The atomic number of each element increases by one, reading from left to right. Blockelements are organised into blocks by the orbital type in. Boron has a charge of 5. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron. Does Boron Share Electrons.
From www.shutterstock.com
Vector Diagram Arrangement Electrons Boron Atom Stock Vector (Royalty Does Boron Share Electrons Borax ore (known as tincal); Blockelements are organised into blocks by the orbital type in. In the second period elements, the two electrons in. In order to achieve a stable electron configuration, boron can either lose three electrons or gain five electrons. Boron has a charge of 5. With its high ionization energy, low electron affinity, low electronegativity, and small. Does Boron Share Electrons.